MIF as therapeutic target
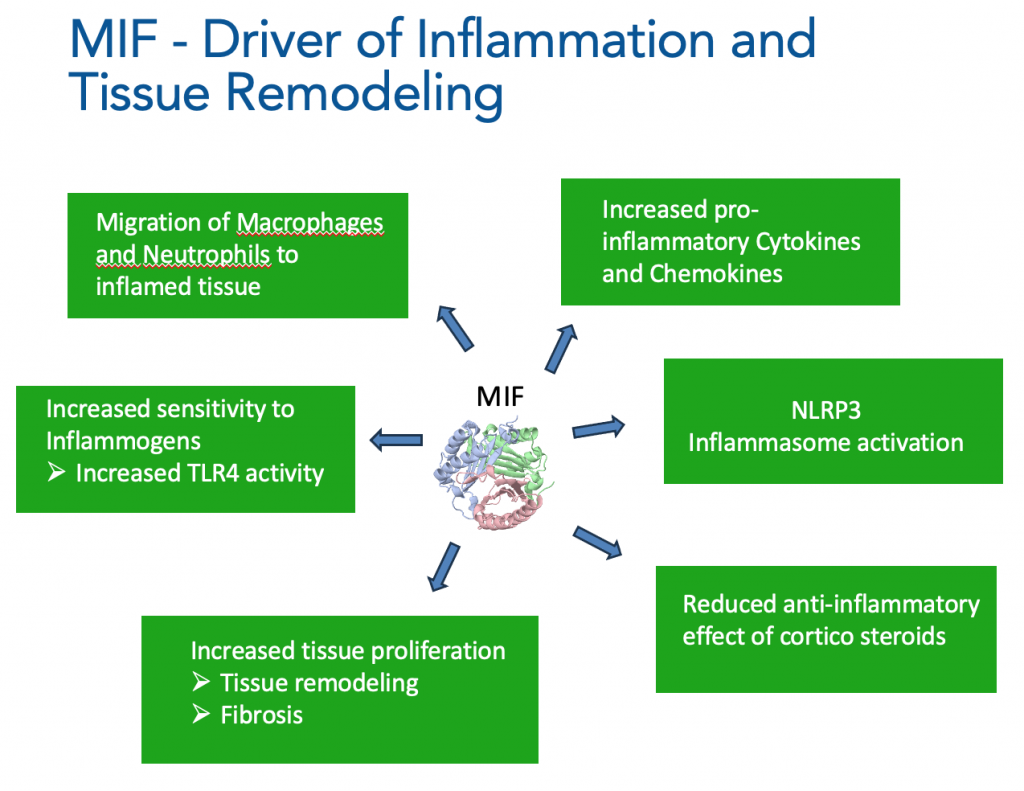
MIF is a cytokine / growth factor-like protein that regulates response to trauma and stress as part of the innate immune system. In acute situations MIF levels increase, which is important to both protect tissue (by blocking apoptosis) and to drive inflammatory responses.
However, prolonged high MIF expression can result in tissue remodelling, fibrosis and chronic inflammation.
MIF as therapeutic target
BLOCKING MIF ENHANCES APOPTOSIS, REDUCES TISSUE REMODELLING AND FIBROSIS.
By binding to the CD74 receptor complex MIF initiates a signaling cascade through activation of MAPK/ERK1/2, AKT (protein kinase B) and NF-κB signaling pathways, leading to proliferation and survival of many cell types, such as fibroblasts and macrophages. Blocking MIF enhances apoptosis and reduces fibrosis and inflammation.
Blocking MIF also inhibits the release of pro-inflammatory cytokines such as IL6 and TNF-alpha.
BLOCKING MIF STOPS MIGRATION OF MACROPHAGES AND NEUTROPHILS TO INFLAMED TISSUE
Binding of MIF to CXCR2 and CXCR4 enhances recruitment of immune cells such as macrophages and neutrophils to inflamed tissue. Immune cell migration is inhibited by blocking MIF.
NLRP3 INFLAMMASOME INHIBITION BY BLOCKING MIF
One of the effects of blocking intracellular MIF is to inhibit NLRP3 inflammasome activation by blocking the interaction of intracellular MIF and NLRP3 units, which is required for NLRP3 inflammasome construction and activation (Harris et al. Nature Comms 2020). NLRP3 inflammasome inhibition by MFC-1040 has been shown to be highly effective in in-vitro and in-vivo models of disease.
NLRP3 Inflammasome inhibition, especially in combination with the other anti-inflammatory and anti-fibrotic effects of blocking MIF promise anti-inflammatory efficacy with improved efficacy compared to single cytokine inhibitor antibodies, such as anti-TNF alpha and anti-IL1 beta.
INHIBITING MIF ENHANCES SENSITIVITY TO CORTICOSTEROIDS
MIF plays a unique role in regulating steroid biology. Whereas steroids reduce activity of other cytokines, steroids stimulate the release of MIF, which subsequently reduces the anti-inflammatory effects of steroids. Consequently, MIF plays a role in the development of steroid resistance. MIF inhibition could therefore result in reduced corticosteroid dosing and reversal of steroid resistance for the many patients that depend on corticosteroid therapy for treatment of their inflammatory or auto-immune disease.
